- Some vaccine-related side-effects are more common than others.
- We look at what the latest data has to say about mild side-effects and those which are more life threatening, such as blood clots and allergic reactions.
- South Africa’s Health Products Regulatory Authority is keeping track of possible side-effects from COVID jabs with its Med Safety App.
In less than a week, on May 17, the health department says South Africa will start to vaccinate people aged 60 years and older, as well as 700 000 healthcare workers not covered by the country’s COVID vaccine implementation study, Sisonke.
Two jabs will be used: Pfizer’s vaccine in metros and Johnson and Johnson’s (J&J) shot in rural districts.
In early May we received our first batch of just over 300 000 Pfizer vaccines. Samples of those jabs are currently being examined by the National Control Lab in Bloemfontein to ensure they’re of an adequate standard.
South Africa has not yet received any J&J vaccines; according to the health department we’ll receive 1.1-million doses in mid-May if there are no delays with the release of the jabs by Aspen’s plant in Gqebera, which is helping J&J to put the vaccine in vials, and to package and label them.
- READ MORE: When will you get your COVID jab? Find out
But how will the Pfizer and J&J shots affect you — can you, for instance, expect any side-effects?
The Sisonke trial, which uses the J&J jab, was, for example, recently paused for two weeks to investigate reports about rare blood clots linked to the vaccine. After it reviewed safety data relating to the jab, the South African Health Products Regulatory Authority (Sahpra), found the J&J vaccine safe for use, but there are now new rules vaccinators have to follow, such as intensified screening for people at high risk of developing blood clots and more detailed follow-up post vaccination.
So how do you know if a side-effect is something to be concerned about and how often are such symptoms likely to occur?
We break it down.
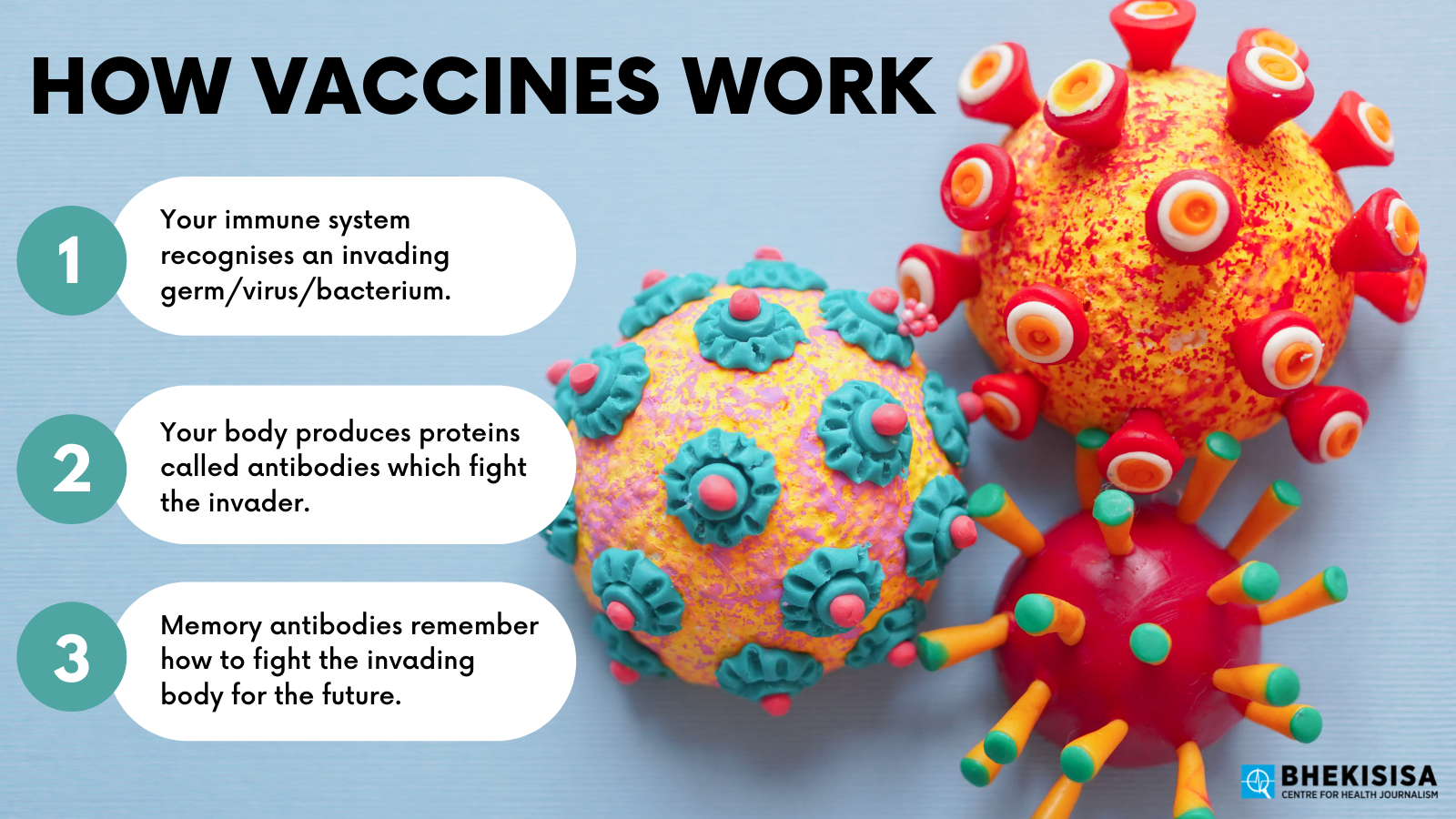
1. What is a side-effect?
To answer that, let’s take a quick refresher on how vaccines work. (A great place to start is to watch the first session of the Vaccine Science 101 course Bhekisisa and the Centre for Evidence-based Healthcare hosted in March.)
Most vaccines introduce a harmless version of a particular germ (in the case of COVID it’s the SARS-CoV2 virus) into your body that triggers your natural defence mechanisms. Your body responds just as if the threat was real — but without you actually getting sick. Some vaccines, such as those using mRNA technology, like Pfizer’s shot, use man-made genetic material that instructs your body how to defend itself against a particular virus.
This “response” consists of your immune system producing antibodies specific to the virus or genetic material that was introduced. Once those antibodies have been produced, your body remembers how to make them, and when you’re exposed to the actual, harmful version of the virus, you’re able to fight it off.
Side-effects are really just a part of your immune system’s natural reaction to a vaccine.
Experiencing a reaction to vaccination helps signal that your body is building immunity against the virus. Not experiencing a reaction, however, is normal too.
Information about a drug or vaccine’s possible side-effects — scientists also refer to this as adverse events — is collected during clinical trials that test the safety and efficacy of a jab. But the data that can be collected during such studies is limited. Firstly, Sahpra cautions, the studies don’t run for long enough to detect long-term side-effects. The trial participants are also far fewer (generally in the thousands) than the millions of people who will eventually receive the jab, which means that very rare side-effects are often not picked up during such studies, because not enough people receive the shot for rare adverse events to show up.
Sahpra has put several mechanisms, such as an app, a hotline and electronic reporting systems, in place to monitor the safety and efficacy of the shots that will be used in South Africa’s COVID vaccine roll-out.
2. So what can you expect after your COVID shot?
Side-effects range from mild to potentially life threatening and some are more common than others.
A study published in The Lancet in April analysed self-reported side-effects from people in the United Kingdom who received either Pfizer or AstraZeneca jabs. The researchers used data from the COVID Symptom Study App, which vaccinated people in the UK can use to report vaccine-related side-effects.
So far, this is the largest study investigating the side-effects of COVID jabs outside of a trial setting. The researchers focused on two types of symptoms: local effects (they occur at the site of injection) and systemic effects (they affect your whole body).
Sixty-six percent of the vaccinated people who used the app between December and March reported experiencing local after-effects such as pain, swelling and tenderness at the site of injection. About one in four people declared side-effects that affected their whole body, such as nausea, fatigue and diarrhoea.
The study found that fewer side-effects were recorded in the “real world” than under clinical trial settings.
How common were side-effects for the Pfizer vaccine?
The COVID Symptom Study App data included 627 383 app users in the UK between December and March. This included 345 280 users who received a first dose of the AstraZeneca jab, 282 103 users who got their first dose of the Pfizer jab and 28 207 users who got a second shot of the Pfizer vaccine.
Because South Africa will be using the Pfizer jab, we’re showing you how the trial data of this jab compared to that of the COVID Symptom Study App:
- 21 720 participants in the Pfizer phase three clinical trial (this is the final phase of the study which tested the safety and efficacy of the jab) received two shots each, three weeks apart.
- After the first dose of the jab, 71–83% of the trial participants reported pain at the injection site; 34–47% reported fatigue and 25–42% a headache.
- After the second dose, 51–59% of participants reported fatigue.
- COVID Symptom Study App data found that less than 30% of app users complained of injection-site pain (compared to 71-83% in the trial), and less than 25% of fatigue (compared to 34-37% in the trial) and headache (compared to 25-42% in the trial) after the first dose.
- App data found that less than 15% of participants reported fatigue after the second dose (compared to 51-59% in the trial).
Local and systemic side-effects — which are sometimes also referred to as mild to moderate side-effects — are generally the more common consequences that you can expect after receiving your COVID jab.
They are a normal part of the vaccination process and people ordinarily recover from such side-effects within a few days.
In South Africa’s Sisonke trial, 2.2% of healthcare workers who received the J&J vaccine had reported “side-effects or an adverse health event following vaccination” by April 12, the researchers explained in a press release. “Most of these events have been minor, local or systemic reactions. One person experienced a severe allergic reaction that met the international diagnostic criteria for anaphylaxis (a severe, potentially life-threatening allergic reaction) but has since made a swift and complete recovery.”
3. More unusual effects like anaphylaxis and briefly blood clots
Most people will experience very mild symptoms or no side-effects at all. But occasionally, COVID vaccines can have a more severe effect for which you may need hospitalisation.
Only two such side-effects have been recorded: a severe allergic reaction (or anaphylaxis) and unusual blood clots.
- Blood clots
Cases of rare types of blood clots, known as cerebral venous sinus thrombosis (CVST), were reported in people who got the AstraZeneca jab in parts of Europe and those who got a J&J’s jab in the United States.
In the general population, CVST affects about three to four adults in one million every year. About one in a million people who received the J&J jab in the US, developed CVST, according to the US government’s Centres for Disease Control’s (CDC) Vaccine Adverse Event Reporting System (VAERS). The clots are, however, more common among women between the ages of 18 and 49 — for them, the CDC found, the risk is about seven out of a million.
Here’s a look at how often blood clot events were observed in different scenarios among people who got COVID vaccines.
- 14 cases of CSVT clots were reported after 8-million doses of J&J jabs administered in the US between March and April, according to a CDC paper.
- By 12 April, three US cases of these clots were observed following 84.7-million Moderna doses, a VAERS report shows. The CDC cautions, however, that VAERS data alone can’t prove the event is linked to the vaccine.
- No incidents were reported among people who were immunised with Pfizer’s vaccine in the US according to the 12 April VAERS report.
Read more about these blood clots and their link to COVID vaccines in our analysis here.
- Anaphylaxis
Anaphylaxis is a severe, potentially life-threatening allergic reaction, which could include skin irritations (hives, itching etc), lowered blood pressure, trouble breathing and nausea.
Although allergic reactions are more common than blood clots, they occur less frequently than mild and moderate reactions, such as a fever. In February, the CDC reported about 4.5 cases of anaphylaxis for every one million Moderna and Pfizer doses administered (each jab requires two doses).
A study published in the Journal of the American Medical Association in March found that about two-thirds of people who experienced severe allergic reactions after receiving a Moderna or J&J jab, had an allergy history — and significantly more women than men had allergic reactions to the shots. On average, an allergic reaction appeared within 17 minutes after someone was vaccinated.
Only 1.7% of app users in the COVID Symptom Study who received a Pfizer or AstraZeneca jab reported experiencing some form of allergic reaction. These included burning skin, rashes and welts on the lips and face.
The study’s researchers found that participants younger than 55 were more likely to experience at least one form of systemic effect compared to app users older than 55.
People receiving a COVID jab are advised to wait at their vaccination site for 15 minutes to see if they develop an allergic reaction. People with a history of severe allergic reactions should wait for 30 minutes before leaving, according to the CDC. Those who have a known allergy to one of the vaccine ingredients should not be immunised with a jab that contains those substances.
4. How South Africa’s is monitoring vaccine safety
In April, Sahpra launched an app to help with the reporting and monitoring of drug and vaccine-related side-effects. Much like the COVID Symptom Study App, Sahpra’s Med Safety App allows users to self-report suspected adverse reactions from medicines they’ve taken. The app also enables Sahpra to provide feedback to users, for instance, safety updates and information about regulated medicines and vaccines.
The Med Safety App was developed by the European Union’s Innovative Medicines Initiative and is supported by the World Health Organisation. The app has also been rolled out in other low- and middle-income countries such as Burkina Faso and Zambia.
A call centre, which will be in operation by 17 May for the launch of phase two of the vaccine roll-out, will be set up so that people who have been vaccinated can use it to report concerns or symptoms that they’ve experienced.
Gopolang Makou was the impact and engagement officer at Bhekisisa.





