- New research from South Africa’s genomic surveillance network shows that people infected with the 501Y.V2 variant are also immune to other forms of the virus.
- The team found the protection against the 501Y.V2 variant could also extend to the original virus circulating the country and the variant first identified in Brazil.
- Understanding the immune response to the new form of the virus in South Africa can help shape how vaccines are designed for more long-lasting and wider protection.
There’s something special about the body’s immune response to the 501Y.V2 variant which is the main form of SARS-CoV-2 (the virus that causes COVID-19) circulating in South Africa. People who are infected with this variant are also immune to previous forms of the virus, South African researchers announced in a recent media briefing.
We draw out key moments from the briefing by a collective of scientists, the Network for Genomic Surveillance in South Africa (NGS-SA). We outline how the researchers discovered this and why this new information about COVID-19 variants could hold the answer to better vaccines.
What is genomic sequencing — and why does it matter?
The coronavirus, like other viruses, is made up of a string of genetic code. Scientists try to unravel that code to better understand how the virus works through a process called sequencing. By regularly sequencing samples from COVID tests done in the country, researchers can paint a picture and track how the virus is spreading and evolving.
Put simply: Genomic sequencing allows researchers to decode the genes of viruses and monitor how they change over time.
What is genomic sequencing?
In South Africa, this effort is being led by a collective of scientists called the Network for Genomic Surveillance in South Africa. It was through this network that a genomics team at the KwaZulu-Natal Research Innovation and Sequencing Platform (KRISP) was able to identify a new variant in the country — called 501Y.V2.
The genomic sequencing conducted by organisations like KRISP helps us understand what the COVID pandemic looks like in South Africa and other countries.
The group was regularly collecting samples of the virus from COVID tests being done by the National Institute of Communicable Diseases (NICD). The NICD serves as a sort of central hub that stores all the samples for the country. This was part of routine sequencing they began when the first case of COVID was detected in the country on 5 March 2020.
Since then South Africa has sequenced just over 3 000 genomes (virus samples).
Here’s what scientists have learned from decoding the genes of SARS-CoV-2:
During a briefing on 3 March, KRISP’s director Tulio de Oliveira presented the team’s data.
In the span of one year, from February 2020 to February 2021, they had completed 3 324 sequences — showing the distribution of coronavirus variants across South Africa.
This helped chart how 501Y.V2 started becoming the dominant variant of the coronavirus in South Africa from 28 September 2020.
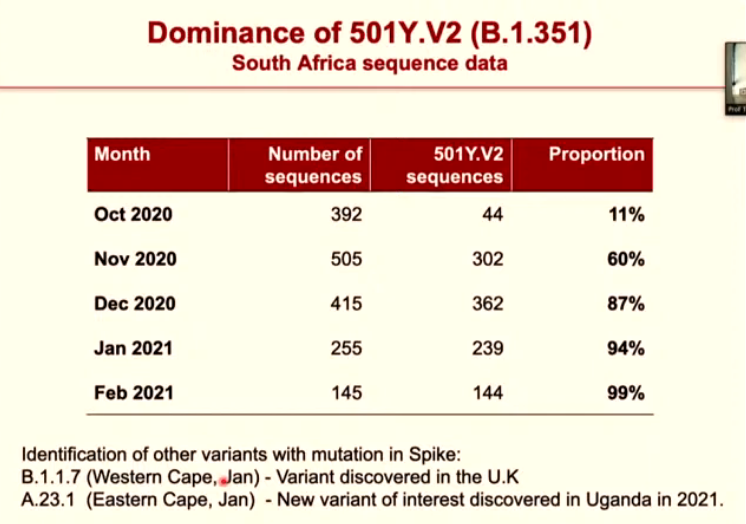
Further analysis of the country’s COVID samples helped show how this new variant slowly began to dominate our epidemic — with the 501Y.V2 samples increasing month by month. This new form of the virus has gone from comprising just over 10% of samples in October 2020 to almost 100% of samples in February 2021.
With the help of this type of analysis we now know that the variant has spread to almost 50 countries worldwide.
How much protection do we have against the new variant?
The 501Y.V2 variant first identified in South Africa is unique because of the number of mutations (or changes to its structure). The variant was unusual not just because of the large number of changes it possessed, but also because of the significance of these changes.
A key site at which the virus attaches itself to human cells (when it infects someone) was altered in a way that helps 501Y.V2 to both spread more easily and evade antibodies produced by the body’s immune system.
Antibodies are our bodies’ first line of defence against most foreign invaders, like viruses. These proteins can help our bodies identify and ward off infections from a virus — this is called immunity. Antibodies form one part of this immunity, as they help our bodies to recognise the structure of a virus and flag it as something that needs to be destroyed.
The problem: The variant is able to outsmart these defences and this means even if you have previously been infected with the original virus, you could get re-infected with the variant.
But there’s a silver lining.
The new variant is more complicated for the body to fight off because of all the changes but your immune system is also quite smart. It can design antibodies to defend itself against this new invader and these will be stronger than the first troops sent into the fray.
As part of the March briefing, researchers in the national genomic surveillance network announced new promising results about how the emerging variants could be fought off or neutralised.
Neutralising a virus means that your body is able to create a sufficient defence against the invader. It does this by stopping the growth and replication of a virus through antibodies.
Sandile Cele, a PhD candidate working at the Africa Health Research Institute (AHRI), studied how effective antibodies from the original version of the SARS-CoV-2 virus were at stopping the virus from infecting cells compared to antibodies developed from infection with the 501Y.V2 variant.
The study found that antibodies from the original virus worked well at neutralising the original virus and similarly antibodies from the 501Y.V2 variant were effective at neutralising the new version of SARS-CoV-2.
This is not surprising given that antibodies are designed to fight off a specific virus, or pathogen, making them a tailor-made response. But it is still reassuring to see the coronavirus following normal rules, noted De Oliveira during the presentation.
Cele and his colleagues then decided to test what happened if the antibodies were swapped across viruses. In other words, if the immune response to the original virus would work against the new variant or vice versa.
So what did they find?
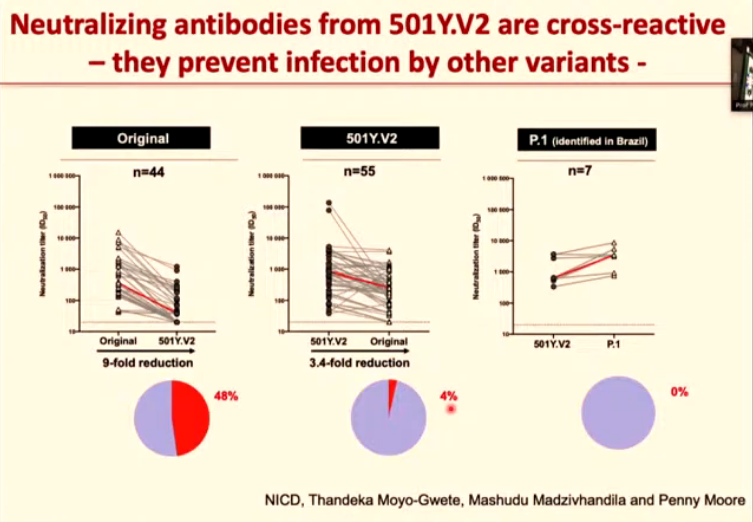
Excitingly, the antibodies that were produced in response to the 501Y.V2 variant could also block other variants.
Antibodies from the original virus couldn’t stop the 501Y.V2 variant from spreading. But antibodies that had been designed to fight off the variant were able to neutralise the original virus to a certain extent.
So the study found that antibodies from the 501Y.V2 variant could neutralise both the original virus and the 501Y.V2 variant. And it also showed that 501Y.V2 antibodies could fight off another variant — 501Y.V3 — which was first identified in Brazil.
These findings were confirmed by data from a separate study by researchers at the NICD.
How South Africa’s genomic experts worked together to find answers
Remember the coronavirus outbreak at Netcare’s St Augustine’s Hospital in April last year?
One patient, infected with SARS-CoV-2, led to the infection of 135 patients and staff. Cases in the hospital complex snowballed to such an extent that by the end of April hospital-related infections made up almost 14% of KwaZulu-Natal’s COVID-19 cases.
One of the main causes for such a rapid spread was the slow rate at which COVID cases were identified at the time, a report published in May 2020 found. KRISP arrived at the scene and began sequencing the virus in people who had been infected at the hospital. It was only with the help of KRISP’s analysis that the hospital was able to track how the virus spread — and in doing so also plan for the outbreak.
Incidents like these help make the case for more collaboration in South Africa’s epidemiological research community, Kholeka Mlisana explained in her presentation at the media briefing where new information on 501Y.V2 was released.
Enter the collaborative efforts of the Network for Genomic Surveillance in South Africa.
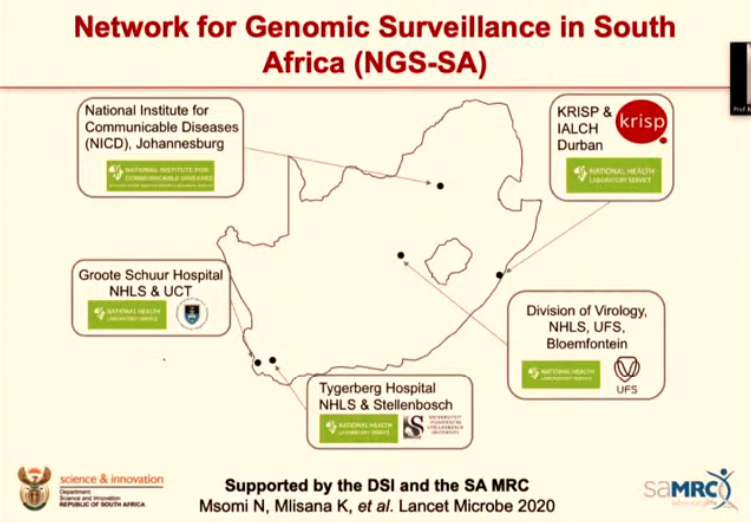
The network includes five National Health Laboratory Service (NHLS) virology labs and nearby academic centres that analyse and monitor genetic data (so they do genomic sequencing).
The benefit: South Africa’s sequencers are not limited to one area. Instead, they can gather a variety of samples from across the country and get a better sense of the big picture and how the epidemic has evolved nation-wide.
Labs partnering with neighbouring sequencing centres allows for speedy and localised analysis. This will help “inform regional and national responses near to where the samples are collected and processed”, Mlisana and her co-authors said in a 2020 article for the Lancet.
By mapping out the versions of the virus in different regions, South Africa can then mount a better response that is tailored to the challenges that area is facing.
How do collaborations like this work?
Let’s take the case of St Augustine’s Hospital as an example.
- St Augustine would submit virus samples to the local NHLS virology lab — in this case the Inkosi Albert Luthuli Central Hospital.
- 50 randomly selected samples, which would be representative of KwaZulu-Natal’s districts, are then forwarded to a sequencing centre such as KRISP.
- Within seven days KRISP would conduct a genomic analysis (or sequencing) of the samples. This includes the processing, measuring and sorting of genetic data such as DNA.
- This type of collaboration now happens across South Africa.
What are the next steps?
The dominant variant in South Africa is unique because of the number of mutations it has. There are also some changes that are common across the various new forms of the virus circulating around the world.
For example, the variants that were identified in Brazil, South Africa and the United Kingdom all share a common mutation — called N501Y — which is why South Africa has adopted a system using this mutation in the name of the 501Y.V2 variant.
The N501Y mutation causes changes to an area of the virus called the “receptor binding domain” (where the virus latches onto our cells). This change allows the spike protein on the virus to more easily attach onto a person’s cells so that the parts better fit together (almost like two puzzle pieces).
Because the variants have a common structural change, antibodies from one variant could potentially help to also neutralise another variant.
Why does this matter?
It helps provide a guide for future research, especially in the field of vaccine development.
The new data that was presented at the briefing shows that antibodies designed to fight off the 501Y.V2 variant have the potential to also stop other variants (like the one circulating in Brazil). Understanding this could help us get a better handle on how vaccines are developed.
Vaccines work by triggering an immune response (in the form of antibodies and killer T cells), even if you have not previously been infected with a virus.
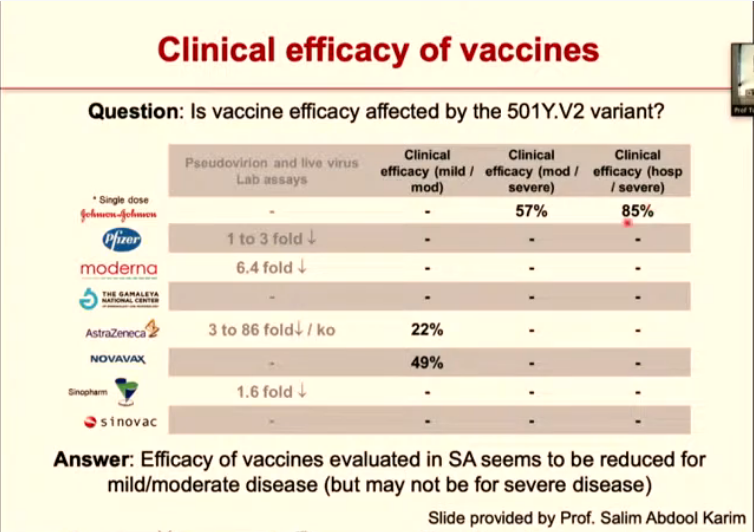
At the moment existing vaccines — including the Johnson & Johnson jab being rolled out in South Africa — have been designed to counter the original form of the coronavirus. The adaptability of the new variants (due to their mutations) means that the vaccines offer less protection against these versions of the virus.
But if we know that antibodies for one variant, in this case the 501Y.V2, can also work against other variants, like the one in Brazil, then it opens the door for vaccines to be designed using variant antibodies.
“Vaccines based on the 501Y.V2 genetic sequence may be more broadly effective,” the researchers found.
This is something already underway with some companies investigating booster shots tailored to the variants and Moderna beginning early clinical trials testing their own 501Y.V2-specific jab.
Understanding how immunity against the new variants works is important because it can guide researchers with regards to the type of vaccines they need to develop. The findings of South Africa’s genomic surveillance network contribute to this understanding and can be used to design jabs that help to limit the risk of re-infection and continued transmission of the virus.




