- Up to half a million Johnson & Johnson vaccines will arrive in South Africa by April. In total, three batches of 80 000 Johnson & Johnson jabs will arrive in the country every two weeks, followed by one batch of 60 000.
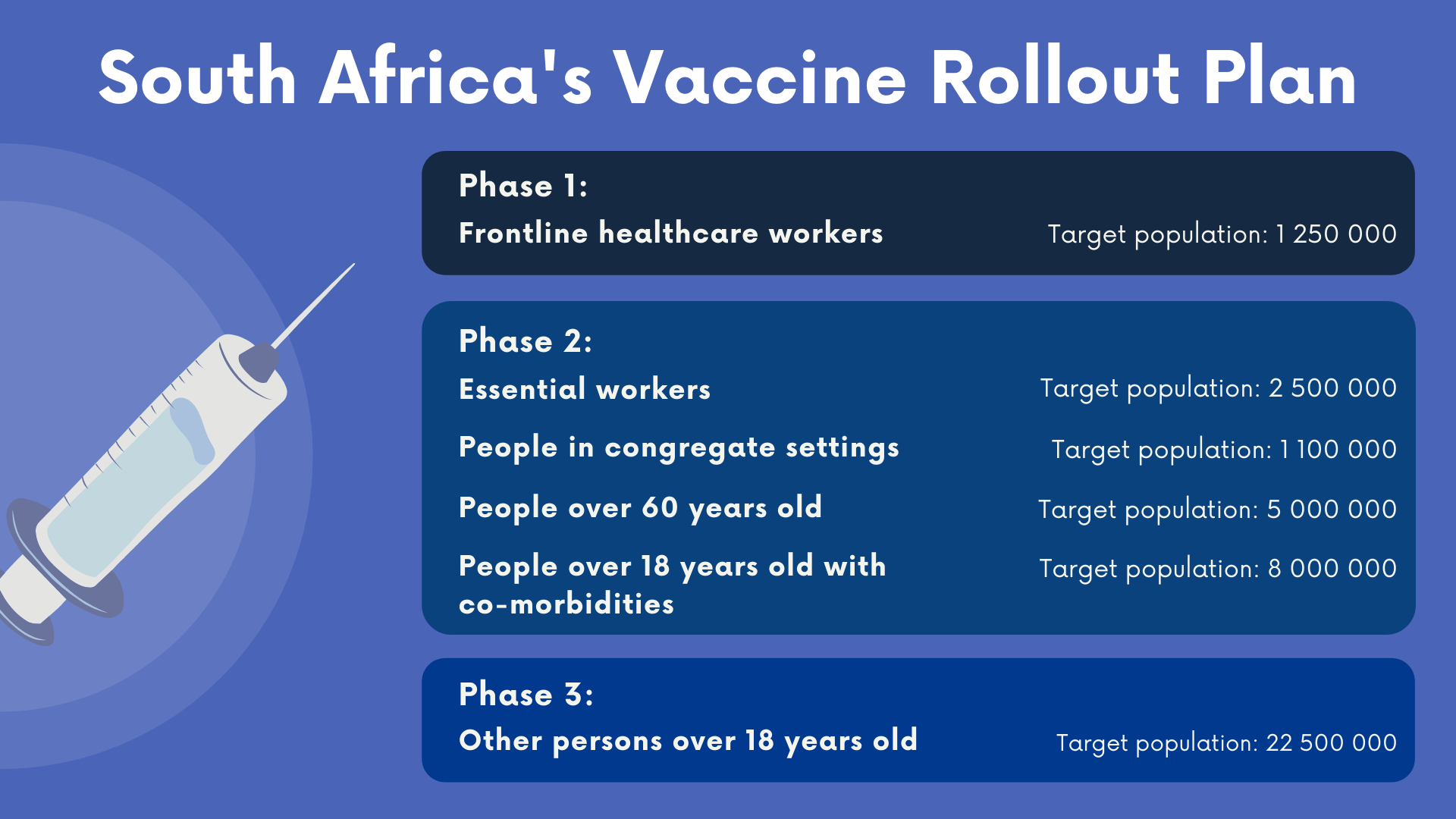
- As of 1 pm on 15 February, around 28% of the 1.25-million healthcare workers who are part of South Africa’s phase one COVID vaccine roll-out had registered on the health department’s online vaccination platform.
- The 80 000 Johnson & Johnson vaccines that arrived on Tuesday night, will be administered at 18 hospitals around the country. Find your province’s hospital with our interactive map.
Eighty thousand Johnson & Johnson jabs touched down at OR Tambo International Airport late on Tuesday night and will be distributed to 16 sites across the country where healthcare workers will be vaccinated.
This marks the start of South Africa’s new, adjusted vaccine roll-out plan where jabs will be given as part of an implementation study conducted by the South African Medical Research Council and the national department of health. The implementation study will focus on “real-life” environment roll-out issues and also gather additional data on side effects and the protection the Johnson & Johnson jab offers against severe COVID-19 disease caused by 501Y.V2, the new SARS-CoV-2 (the virus that causes COVID-19), that is now dominant in South Africa.
We break down everything you need to know about the Johnson & Johnson vaccine — how well it works against the new variant, how many doses will be going to which sites (check out our interactive map), and how many more vaccines will be arriving and when.
1. How well does the J&J vaccine work?
The Johnson & Johnson vaccine is the only jab — at least so far — for which we have clinical trial data on the kind of protection it provides against severe COVID-19 disease caused by the new variant, 501Y.V2.
Why are we interested in protection against severe disease? Because that’s the type of COVID that leads to people ending up in hospital or dying. Reducing these cases means our health system doesn’t get overwhelmed, and the impact of the pandemic on our economy when breadwinners, for instance, die, is alleviated. In the case of health workers, who are on the frontline taking care of people with severe COVID and therefore most vulnerable to infection, we’d want to protect them as much as possible.
Johnson & Johnson tested their vaccine in eight countries — the United States, Argentina, Brazil, Chile, Colombia, Mexico, Peru and South Africa. Fifteen percent, or 6 567 of the study’s 43 783 participants, were from South Africa.
Most trial participants were older than 60 years (33.5% over 60, 19.6% over 65, 3.5% over 75, 1% over 80). This helped the researchers to get better data on how the vaccine fared against severe COVID (because people above 60 are more likely to get severe COVID than those who are younger than 60).
The study showed that the jab was 85% effective in preventing severe disease in people who were 18 years and older in all the countries that were studied. The protection kicked in 28 days after the shot was given. What is more, is that the protection the jab provided against severe disease increased over time — 49 days after a jab was given, not a single case of severe COVID was reported in people who had been vaccinated.
Moreover, the jab demonstrated 100% protection against COVID-related death: 28 days post-vaccination, no trial participant had died.
So how do the study’s investigators know that the COVID cases in the South African arm of the trial were caused by 501Y.V2? They took a sample of the cases and sent them for something called genomic sequencing, which allowed them to decode the genes of the virus and establish which variant it was. The results showed that about 94% of the COVID cases from South Africa were caused by the variant, one of the two co-principal investigators of the local Johnson & Johnson trial, Glenda Gray, said in a presentation on 7 February. Gray is also the president of the South African Medical Research Council.
But the trial data also showed the study’s researchers that the Johnson & Johnson vaccine offers less protection against moderate to severe COVID caused by 501Y.V2 than against severe COVID. The jab offered 57% protection against moderate to severe disease in South Africa, which was lower than the protection provided in countries where the 501Y.V2 variant was not dominant. The 57% is, so far, however, the best protection we’ve got in a vaccine against moderate to severe disease caused by 501Y.V2 — both the Novavax and AstraZeneca jab showed less than 50% protection, which is the World Health Organisation’s threshold for a COVID jab to be considered effective.
2. Has the data been peer-reviewed?
Before a scientific study can be accepted for publication in a reputable academic journal, the research goes through a process called peer review. This means a committee of “peers”, in other words, other academics or researchers, examine the data and methodology of the study, to make sure it is sound.
This process can take months to complete.
During the COVID pandemic, research moved considerably faster than usual, because of the need to find treatments and vaccines fast. This resulted in study results often being released in the form of preprint studies (that have not been peer-reviewed) or press releases.
In the case of the Johnson & Johnson vaccine, the study results have not been peer-reviewed. The data was released in the form of a press release on 29 January. This means there is limited data available about the exact numbers being looked at and we don’t yet know how many of the COVID cases that were recorded during the trial occurred in which of the eight countries that the trial was done.
3. How many shots do you need?
So far, the Johnson & Johnson jab is the only COVID vaccine that requires only one shot. All the other jabs that are currently available, require two shots. This makes the Johnson & Johnson vaccine considerably easier to administer because health workers don’t have to spend time and resources on ensuring that people return for their second shot.
Potentially, later down the line, researchers could test if a second “booster shot” designed against the 501Y.V2 variant, could increase the protection provided.
4. How many Johnson & Johnson doses are we getting — and when?
For now, we’ll be receiving four batches of vaccines, once every two weeks, says Linda-Gail Bekker, the other co-principal investigator of the Johnson & Johnson study. The first three batches will consist of 80 000 doses, and the fourth one of 60 000 doses. Once we’ve received 300 000 doses (the total of the first four batches) and showed that we were able to administer all of them, we will have the option of asking Johnson & Johnson for another 200 000 doses.
All these doses (500 000 in total) will be provided to South Africa for free by Johnson & Johnson. Why? Partly solidarity, and partly because they come from research stock that Johnson & Johnson won’t be using, says Bekker.
In addition to the free vaccines, South Africa has procured nine million doses from Johnson & Johnson, which, according to a January presentation of President Cyril Ramaphosa, is expected to arrive in the second quarter of this year. Media reports on 9 February stated that an additional 20-million doses are under negotiation, but this has not been officially confirmed.
For these doses, South Africa, will, however, need to pay. And when we get these will depend on two things: when Johnson & Johnson has stock available, and when our medicine regulator, Sahpra, is able to conclude Johnson & Johnson’s application for approval to use the vaccine for a wider roll-out (the approval we got for the first 500 000 doses is for use in a research setting, which is different from the type of approval required for wider use, and also a much simpler and quicker process).
5. When will the roll-out start?
Bekker says, “if all goes well, we’ll start with the first vaccinations by midday on Wednesday, 17 February”.
6. Who is getting the vaccine?
Only healthcare workers can get vaccinated for now — until all of the country’s estimated 1.25-million health workers have been covered. Health workers are considered the most vulnerable to infection because they deal with infected patients daily. That is why they are the only group targeted in phase one of South Africa’s vaccine roll-out.
The first batch of 80 000 vaccines will be distributed to 18 sites across nine provinces, says Bekker, who is also the director of the Desmond Tutu HIV Centre at the University of Cape Town.
One site (Pelonomi Hospital in the Free State) was however not ready to vaccinate when the roll-out began but has now been phased in, the health department’s spokesperson, Popo Maja, told Bhekisisa. An additional site (Khayelitsha District Hospital) has been added to the Western Cape, meaning there are now a total of 18 sites across the country.
Each province has two vaccination sites — except for the Northern Cape with one and the Western Cape with three — all are public hospitals.
So how many doses of the 80 000 doses will each site get?
Bekker says the numbers will be calculated based on the number of trained staff available at a site and the burden of disease (the number of infections in the area). “Additional training will also be done to increase the amount of people able to administer the jab beyond just healthcare workers,” she says.
For the second consignment of 80 000 doses, some of the vaccines will be distributed to private healthcare sector sites, which will then be added to expand the list of 18 vaccination sites. As more jabs arrive, the sites will further increase.
Bhekisisa obtained a list from the national health department with the names of the 18 vaccination sites that are ready to operate and the number of doses they will receive. We’ve turned it into an interactive map to make it easy to understand.
7. How do you let the health department know you’d like to be vaccinated?
Everyone who wants to get vaccinated needs to register on an online system called the electronic vaccination data system (EVDS). This system will record your name, ID number, address, whether you have a medical aid, and a few other details. It will then let you know if you’re eligible to be vaccinated. Once you’ve been identified as eligible, you will receive a text message that will tell you where and when you will get vaccinated. Along with that, you will be sent a unique code, that you will have to give to the vaccinator, along with your ID. Once you’ve received your jab, you will receive an electronic vaccination certificate.
Registration for health workers on the EVDS has started. By 1 pm on 15 February, 351 213 (28%) of the country’s 1.25-million health workers had registered on the platform, according to health department data.

8. So why are we doing a research study with Johnson & Johnson’s vaccine if we already have data that shows it works against 501Y.V2?
The short answer is: Because getting a vaccine approved for use in a research setting is considerably quicker than getting it approved for wider use, and if we have to wait for the latter, we’re likely to only start with roll-out in about two months.
The long answer: Johnson & Johnson has submitted a rolling application with the South African Health Products Authority, Sahpra, to enable them to roll-out their vaccine on a large scale. But, according to Gray, the approval process is likely to only conclude in about three months (the review started towards the end of 2020).
This is because Sahpra has to review loads of data to ensure the vaccine is as safe and effective as Johnson & Johnson says it is. Another complicating factor is that, in order to speed up this process during a pandemic, Sahpra makes use of something called a reliance mechanism. This means they heavily lean on the findings of other large regulators, such as the United States’ Food and Drug Administration (FDA) and the European Medicine Agency. But the Johnson & Johnson vaccine is new, so no other country has yet approved it. The FDA has scheduled an independent review for 26 February, but because Sahpra already started to review Johnson & Johnson’s data a few months ago, they are likely to complete their own review without waiting for the FDA’s findings.
So, while Johnson & Johnson is waiting for approval for wider use of their vaccine, they’re doing an implementation study — because it’s the only way that health workers can get earlier access to COVID vaccinations with this jab.
But it will also provide them with more data.
“We’re studying how the vaccine performs under real-world conditions,” Gray explains. “By doing the roll-out in the form of an implementation study, additional information can be gathered to confirm the findings of the phase three study that this vaccine protected against hospitalisation and death.
While there is existing trial data showing the vaccines’ safety, certain groups like pregnant women are not able to participate in clinical trials. “Groups like these that were previously excluded from trials can now participate in the implementation study and provide information on how the vaccine performs and to continue monitoring the jabs’ safety in a wider roll-out,” Gray says.
Bekker concludes: “Additionally, this approach to roll-out can provide insight into vaccine uptake among the population.”
[Updated 14:30 19 February 2021: This article was updated to reflect the addition of two new vaccination sites (one in the Free State and one in the Western Cape).]




